Thibault TROADEC
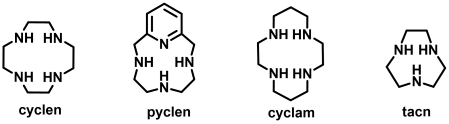
Polyazamacrocycles (cyclen, cyclam, tacn, pyclen) are ubiquitous chelating ligands for a large range of metallic cations, and are particularly used in the design of molecular tracers in medical imaging and nuclear medicine. Over the last two decades, our group has prepared a wide range of optimized chelators, bearing tailored sets of coordinating arms (acetate, picolinate, thiazolyl, pyridyl...) for different metallic cations used in :
- Magnetic Resonance Imaging (MRI) : Gd3+, Mn2+
- Positron emission Tomography (PET) : 64Cu, 68Ga, 89Zr
- Internal Radiotherapy : 67Cu, 212Pb, 213Bi, 109Pd
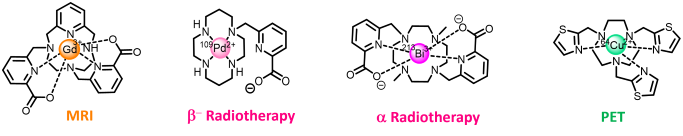
Tripier et al. (MAC Research group) :
Chem. Commun., 2014, 12371
Inorg. Chem. 2019, 58, 2669
Inorg. Chem., 2021, 60, 2390
Chem. Eur. J., 2022, e202200942
Polyazamacrocycles and heteroelements
My research focusses on the use of heteroelements (P, B, F mainly), that have been somewhat underexplored in the domain, to bring new coordination behaviour and/or new functionnalities to these chelators, and access new properties for applications in the medical domain and beyond.
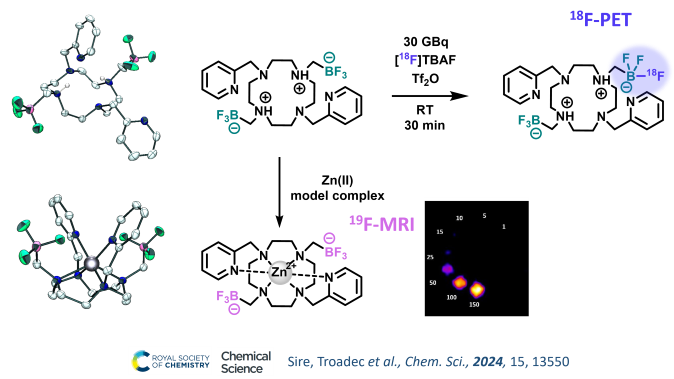
Trifluoroborate (BF3) arms for 18F-PET or 19F-MRI combinations with chelators
Trifluoroborate units are excellent prosthetic groups for 18F-radiolabeling via easy late-stage 19F/18F isotopic exchange in soft conditions. Therefore we are studying the combination of these fluorinated functional groups with azamacrocyclic chelators to access potential theranostic (therapy+diagnostic) probes, with PET imaging allowed by the BF3 units, and therapy by a dedicated cation in the macrocyclic cavity.
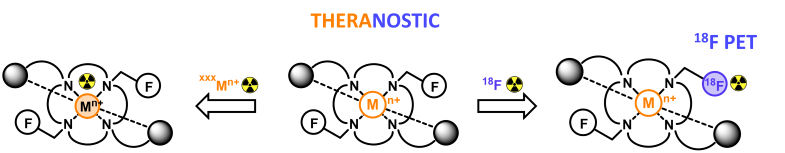
We recently presented the first chelator from this new family, that demonstrated excellent stability and 18F-radiolabeling properties of the BF3 groups. For the first time, we also explored the properties of BF3 groups as 19F MRI reporters, a feature that could open the way towards the design of smart MRI probes.
Phosphorus-based side-arms for new coordination properties
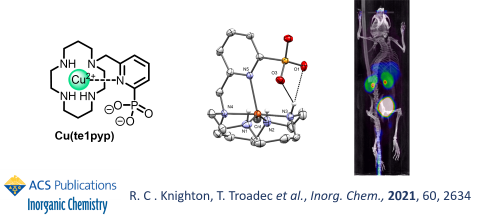
Phosphorus has been previously used in coordinating groups on polyazamacrocycles, mainly under the form of phosphonate or phosphinate functions, which demonstrated excellent properties for fast complexation of radiometals (64Cu in particular) and low undesired accumulation in vivo.
Therefore, we combined these properties with the excellent inertness brought by picolinate (pyridine-2-carboxylate) groups, by designing cyclam chelators bearing pyridine-2-phophonate moieties. These demonstrated excellent properties for 64Cu-radiolabeling and in vivo biodistribution, with minimal accumulation in healthy tissues.
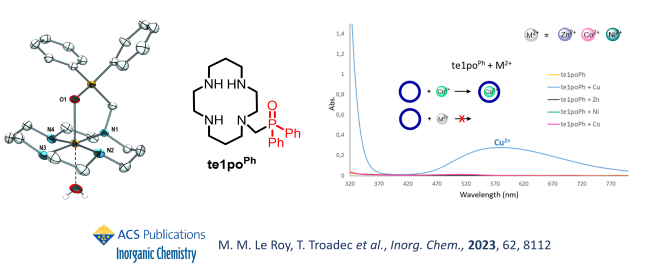
We are also exploring new phosphorus-based topologies, and recently described a novel cyclam chelator bearing the diphenylphosphine oxide motif, that has shown very peculiar specific coordination of copper(II) ions, a very rare behaviour with such chelators.
Structural Modification of Polyazamacrocycles
C,C-bismacrocycles
We are also interested in more synthetical and methodological aspects of polyazamacrocyclic chemistry, with the modification of the carbon skeleton of the chelators. We have for instance developped a new synthetic sequence to prepare a "naked" C,C-biscyclam derivative, that has in addition demonstrated promising properties as an anti-HIV drug.

Org. Biomol. Chem. 2024, 22, 3059
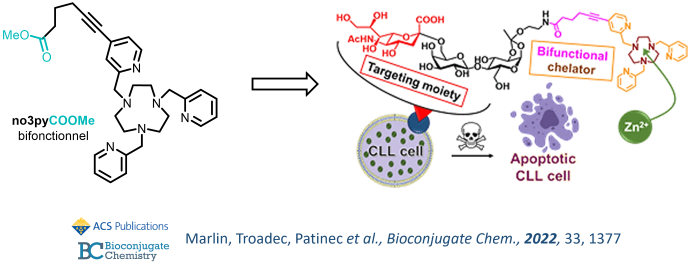
Aryl-functionalization
In order to specifically deliver in vivo the complexes of interest to tumors or other pathological tissues, while retaining the coordination properties of the chelators, our group has developed tools for the modification of the carbon skeleton of the macrocycles for introduction of grafting functions. We are now also developing such strategies on the backbone of aromatic coordinating units (pyridine, picolinate), as a synthetically convenient alternative. This strategy has been applied recently for the design of targeted zinc(II) chelators for treatment of B-cells from Chronic Lymphoid Leukemia.