Group Picture

April 2025
Our Research
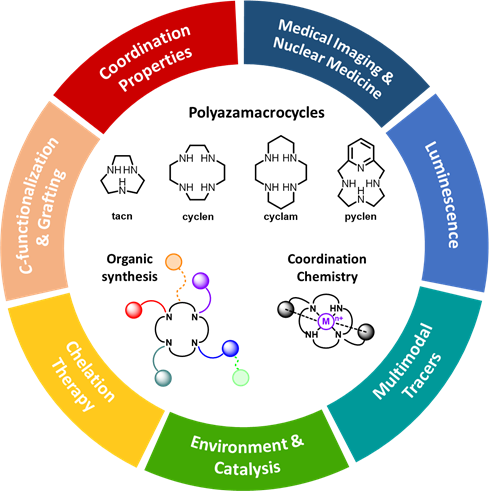
We are interested in the chemistry of polyazamacrocycles, i.e. cyclic compounds containing at least 9 atoms and 3 donors, that have exceptional properties for the chelation of a wide range of cations (transition and post-transition metals, lanthanides). Our core expertise lies in the domains of :
- Organic & macrocyclic synthesis : development of synthetic tools for the regioselective N-functionalization of the macrocyclic platforms, C-functionalization & aryl-functionalization strategies for the preparation of graftable/bifunctional chelators.
- Coordination chemistry : investigation of the chelators properties towards various cations, such as themodynamic association constants (by potentiometry, UV-Vis & NMR spectroscopies), kinetic inertness in competitive media or structural studies (X-Ray diffraction, EPR spectroscopy).
With these tools, we specifically design chelators containing coordinating units to accommodate a designated cation, and additional chemical functions necessary for the desired application in various domains such as medicine, materials, catalysis or environment-directed processes.
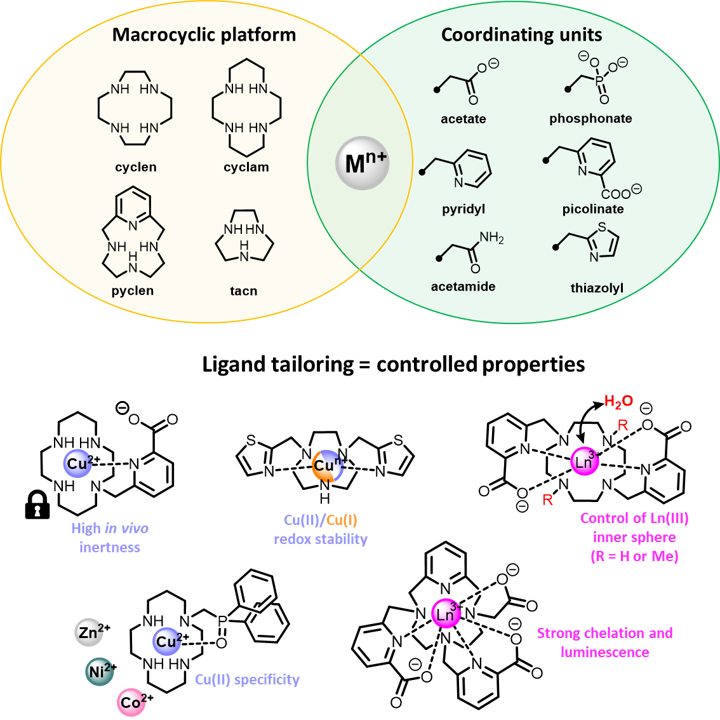
Thanks to a large variety of organic/macrocyclic synthetic tools, we are able to regioselectively functionalize the macrocyclic platforms with selected sets of coordinating "arms" (e.g. acetate, pyridyl, picolinate...) that allow us to finely tune the coordination properties of the chelators. Playing on the denticity and the hard/soft donor character of these ligands thus leads to a fine control of the chelate properties.
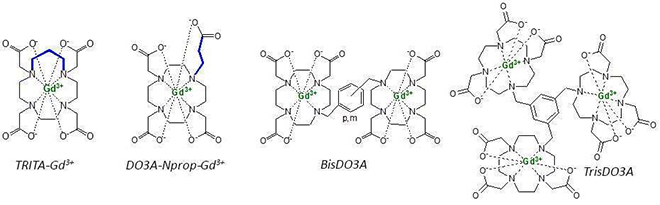
For instance, the use of bidentate picolinate units on the different platforms has lead to highly robust chelators for a wide range of cations, such as copper(II) (Inorg. Chem. 2012) or lanthanides(III) (Inorg. Chem. 2018; Inorg. Chem. 2020). Subtle modification of the chelator substitution pattern can also allow precise control of the inner coordination sphere of the lanthanide cations, with coordination or absence of a water molecule (Inorg. Chem. 2012), a feature that has a key impact in applications such as MRI (Magnetic Resonance Imaging) or luminescence.
The use of less common coordinating arms can also trigger peculiar properties to the chelates, as we have demonstrated for example with the use of thiazolyl groups that provide remarkable Cu(II)/Cu(I) redox stability (Dalton Trans. 2016), phosphine oxide pendants that allow specific coordination of Cu(II) ions versus their divalent congeners (Zn(II), Co(II), Ni(II)) (Inorg. Chem. 2023), or tridentate pyridylphosphonates that lead to the controlled formation of supramolecular constructs with lanthanide ions (Inorg. Chem. 2023; Inorg. Chem. 2020)
Coordination Chemistry
Cationic species complexation: transition and heavy metals, lanthanides. Thermodynamic, kinetic and inertness studies.
Ligand design and complexes properties.
Health :
- Gadolinium(III) cyclen based complexes as MRI contrast agents.
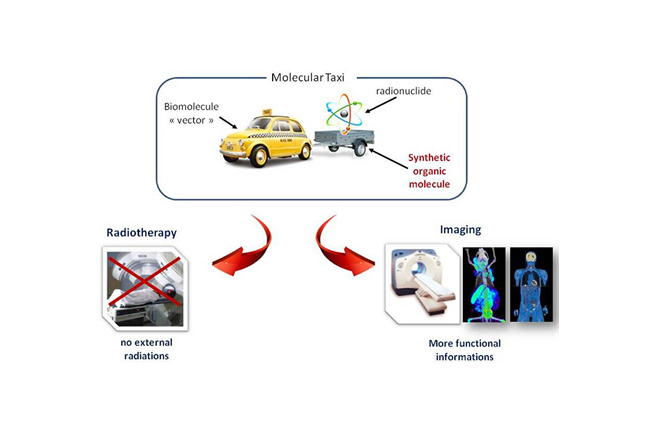
- Copper(II) cyclen-based or cyclam-based complexes for radiopharmaceutical PET imaging applications: synthesis, structural analysis, stability and 64Cu complexation
- Bismuth(III) and Lead(II) complexes for radioimmunotherapy: synthesis, structural analysis, stability and 213Bi and 212Pb complexation.
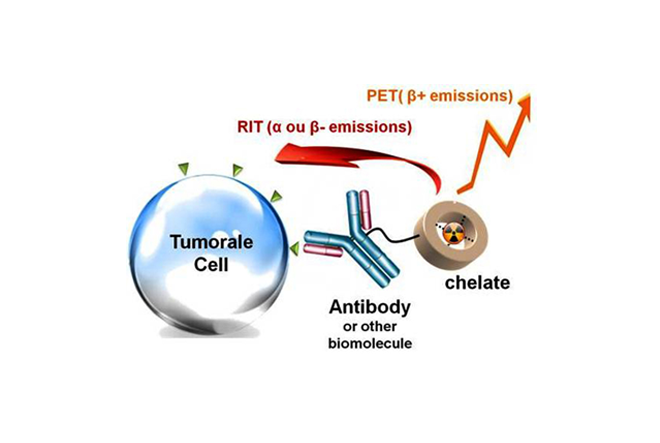
Materials
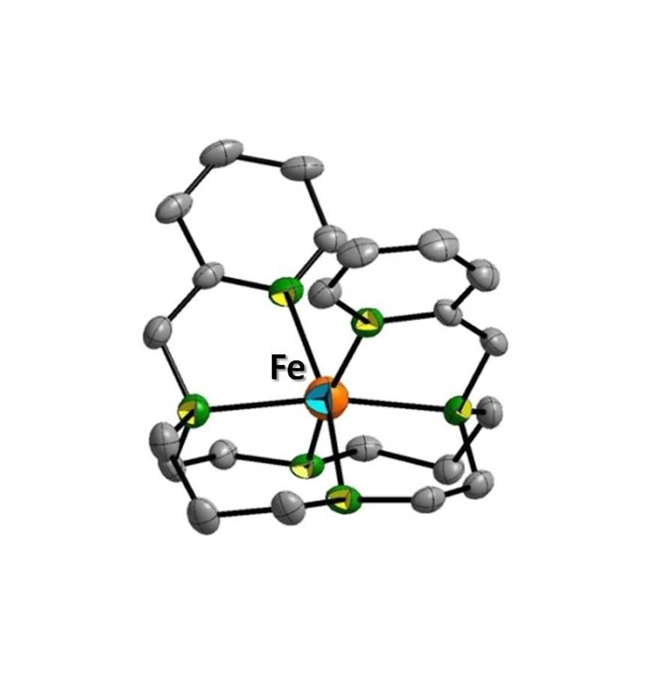
- Macrocyclic aza-ligands for Iron(II) spin crossover systems (coll. with Pr. S. Triki). Cyclen, cyclam and TACN selectively functionalized for new magnetic purposes.
Anionic species complexation: organic and inorganic anions; anions of biological or environmental interest. Phosphates, nucleotides, pollutants…
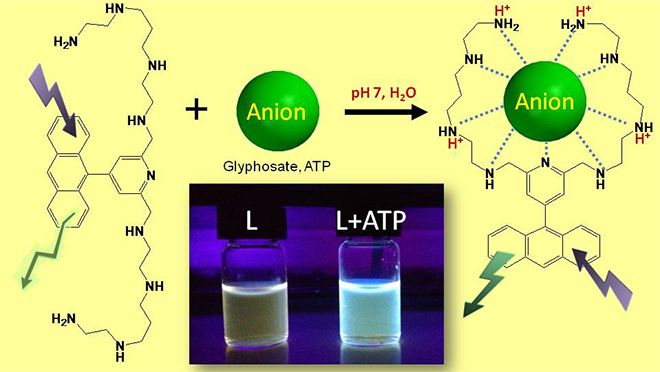
- Supramolecular chemistry of anionic species and in particular the molecular recognition and sensing:
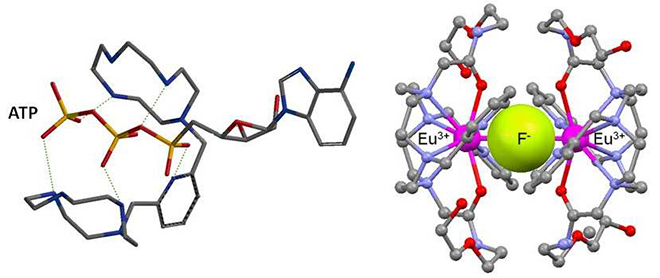
- Host-guest chemistry with polyammonium chelates or metallated azareceptors for phosphates, ATP or glyphosate coordination.
- Cyclen-based lanthanide(III) complexes for fluoride complexation and detection.
Analytical tools :
Thermodynamic and kinetic studies by 1H, 13C, 31P NMR investigations, UV-visible or stopped flow measurements and potentiometric titrations.

Contacts
-
Raphaël TRIPIER
Professeur - Vice-président recherche et innovation - Responsable Groupe thématique "Macrocycles Azoté et Coordination"
raphael.tripier@univ-brest.fr








